Abstract
Introduction: Allogeneic (allo) CAR T cell therapies that produce durable remissions in pts with R/R LBCL may offer advantages over autologous (auto) CAR T products such as the potential for immediate "off-the-shelf” availability, no leukapheresis, an improved safety profile and the option for additional infusions. CTX110® is an investigational allo CD19-directed CAR T cell product engineered using CRISPR/Cas9-editing to disrupt both the endogenous T-cell receptor (TCR) alpha constant (TRAC) locus to prevent TCR expression, and the β2-microglobulin gene to eliminate major histocompatibility complex (MHC) class I expression.
Methods: CARBON (NCT04035434) is a phase 1, open-label, multicenter, global study evaluating the safety and efficacy of CTX110 in adult pts with R/R LBCL with ≥2 prior lines of therapy. Key exclusion criteria included prior auto CAR T and central nervous system involvement. Pts received standard lymphodepleting chemotherapy (LDC) with fludarabine 30mg/m2 and cyclophosphamide 500mg/m2 for 3 days, followed by CTX110. Clinically active doses of CTX110 ranged from 300 x 106 to 600 x 106 CAR+ T cells. Pts who achieved initial benefit and subsequently progressed could receive an additional infusion of CTX110. A subset of pts with an initial Day 28 response of ≥stable disease (SD) were eligible for a second planned infusion on Day 35. Primary endpoints included safety (incidence of dose limiting toxicities, DLTs) and overall response rate (ORR) per Lugano 2014 Criteria. Secondary endpoints included complete response (CR) rate, duration of response and overall survival (OS).
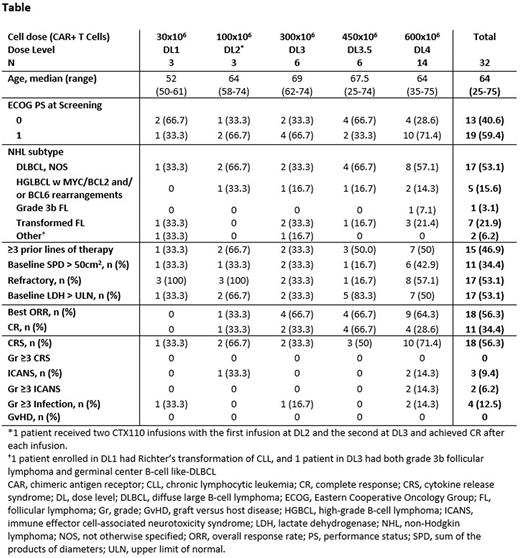
Results: As of April 22, 2022, 34 pts with LBCL were enrolled for dose escalation and 32 received CTX110. Only 2 enrolled pts did not receive CTX110. Median time from enrollment to the beginning of LDC was 2 days. Among pts who received ≥1 infusion of CTX110 at doses of ≥300 x 106 CAR T cells (DL ≥3; N=27), best ORR and CR rates were 18/27 (67%) and 11/27 (41%) respectively (Table); the 6-mo CR rate was 19% (5/27) and there are 2 patients in CR past 24 months with the CR still ongoing in both. For the 8 pts who received a second infusion of ≥300x106 CAR T cells, CAR T cell expansion was observed in all pts, and 5 pts achieved deepened clinical response. Following CTX110 infusion, there was no graft versus host disease (GvHD) nor infusion reactions. Any grade (Gr) cytokine release syndrome (CRS) was reported in 18/32 (56%) pts with no CRS Gr ≥3. Any grade immune effector cell-associated neurotoxicity syndrome (ICANS) was reported in 3/32 pts (9.4%), including 2 cases of Gr ≥3. Gr ≥3 infections occurred in 4/32 pts (12.5%) including 1 pt who died with HHV6 encephalitis. One pt experienced Gr 1 hypogammaglobulinemia after CTX110 infusion. There were 7 pts who experienced serious adverse events that were attributed to CTX110. No increased toxicity was observed with re-infusion of CTX110 and there were no new safety signals observed.
Conclusions: In a heavily pre-treated patient population with R/R LBCL (46.9% with 3 or more prior lines of therapy), CTX110 at DL≥3 or higher resulted in clinically meaningful ORR, CR rates, and durable remissions, accompanied by a favorable safety profile during dose escalation. Nearly half of all patients who achieved a CR maintained it out to at least 6 months. CTX110 offers a potential off-the-shelf treatment option; only 2 enrolled pts were unable to receive CTX110 and the median time from enrollment to LDC was just 2 days, followed by infusion of CTX110 a median of 3 days afterwards. Administration of a second CTX110 infusion was well tolerated and demonstrated evidence of further clinical benefit. Encouraging clinical results have been observed with CTX110 including durable CRs and there is the potential for deepening of responses with additional pre-planned second infusions. CTX110 will continue to be evaluated in an expansion phase of the study.
Disclosures
McGuirk:Novartis: Consultancy, Honoraria; Allovir: Consultancy, Honoraria, Research Funding, Speakers Bureau; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Nextar: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Speakers Bureau; Orca Bio: Research Funding; Sana: Honoraria; CRISPR Therapeutics: Consultancy; In8bio, Inc.: Other: IIT Clinical Trial. Tam:AstraZeneca: Honoraria; LOXO: Honoraria; Beigene: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Riedell:Tessa Therapeutics: Research Funding; MorphoSys: Research Funding; Fate Therapeutics: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Xencor: Research Funding; Calibr: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sana Biotechnology: Consultancy; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees. Ho:Antengene: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Regeneron: Membership on an entity's Board of Directors or advisory committees. Maakaron:CRISPR Therapeutics: Research Funding; Gilead: Research Funding; Precision BioSciences: Research Funding; Scripps: Research Funding; Fate Therapeutics: Research Funding; ADC Therapeutics: Research Funding. Waller:Orca Bio: Research Funding; Verastem Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Awan:BeiGene: Consultancy; Incyte: Consultancy; Verastem: Consultancy; MEI Pharma: Consultancy; Karyopharm: Consultancy; Celgene: Consultancy; Kite Pharma: Consultancy; Gilead Sciences: Consultancy; Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy; AbbVie: Consultancy; AstraZeneca: Consultancy; Cellecter Bisosciences: Consultancy; ADCT Therapeutics: Consultancy; Cardinal Health: Consultancy; Merck: Consultancy; BMS: Consultancy; Dava Oncology: Consultancy; Johnson and Johnson: Consultancy; Epizyme: Consultancy; Genentech: Consultancy; Caribou Biosciences: Consultancy. Shaughnessy:Sanofi: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Kite Therapeutics: Speakers Bureau; Novartis: Consultancy. Ghobadi:Amgen: Consultancy, Research Funding; Atara: Consultancy; CRISPR Therapeutics: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; Wugen Inc: Consultancy; Celgene: Consultancy. Bishop:Immatics: Research Funding; Autolus: Consultancy, Research Funding; Arcellx: Consultancy, Research Funding; WindMIL Therapeutics: Consultancy; Bluebird Bio: Consultancy; Iovance: Consultancy; CRISPR Therapeutics: Consultancy, Research Funding; Agios: Consultancy, Honoraria, Other: Travel support, Speakers Bureau; Bristol Myers Squibb: Honoraria, Other: Travel support, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Travel support , Research Funding; Sanofi: Honoraria, Speakers Bureau; Celgene: Honoraria; Incyte: Honoraria, Other: Travel support , Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel support, Research Funding, Speakers Bureau; Triumvira: Research Funding; Tmunity: Research Funding; Chimeric Therapeutics: Consultancy; Sana Biotechnology: Consultancy; ADC Therapeutics: Speakers Bureau; Servier: Speakers Bureau. Dickinson:Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD, Amgen: Honoraria; Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD: Consultancy; Roche, Novartis, Kite, Gilead, MSD, Takeda, Celgene: Research Funding. Ramakrishnan Geethakumari:Kite: Consultancy; BMS: Consultancy; Rafael Pharma: Consultancy; Pharmacyclics LLC: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Cellectar Biosciences: Membership on an entity's Board of Directors or advisory committees. Ross:CRISPR Therapeutics: Current Employment. Stevens:CRISPR Therapeutics: Current Employment. Xu:CRISPR Therapeutics: Current Employment, Current equity holder in publicly-traded company. Ma:CRISPR Therapeutics: Current Employment. Cohen:CRISPR Therapeutics: Current Employment, Current equity holder in publicly-traded company. Maziarz:Orca Bio: Other: Support for research analysis and for medical writing; Novartis: Other: Support for research on CART; CRISPR Therapeutics: Consultancy, Honoraria; ASTCT: Membership on an entity's Board of Directors or advisory committees; Allovir: Other: Support for research on Allo HCT costs of care of infectious related complications.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal